Project Summary
Toward an Understanding of the Pre‐harvest Ecology and Approaches to Control of Salmonella
- Principle Investigator(s):
- G. H. Loneragan1, T. S. Edrington2, D. M. Brichta‐Harhay3, K. J. Genovese2, D. J. Nisbet2
- Institution(s):
- 1Department of Animal and Food Sciences, Texas Tech University
- 2United States Department of Agriculture, Agriculture Research Service, Southern Plains
- 3United States Department of Agriculture, Agriculture Research Service, U.S. Meat Animal Research Center
- Completion Date:
- May 2013
Background
Because peripheral lymph nodes (PLN) are internal, they escape the usual surface‐focused antimicrobial interventions utilized during the slaughter process. Unless removed during trimming, they may constitute a significant source of Salmonella in ground beef, which might pose a food safety risk.
Given the challenge of PLN removal, solutions will need to be implemented on the pre‐harvest side of production. If this is to be accomplished, the principle that prevalence is a simple function of both the incidence of infections (i.e., the rate of new PLN infections per unit time) and the duration of infection (DOI; i.e., time) should be explored. Observing incidence is problematic or even impossible in field settings. However, if incidence is held as a constant, DOI can be estimated. Using an experimental model of infection developed in part by Beef Checkoff support, incidence can be controlled, and the DOI observed. Given this knowledge, incidence can, therefore, be estimated using the observed prevalence and DOI. What remains unknown, however, is the variation in prevalence across a variety of PLN within a carcass.
The objectives of this study were to 1) evaluate within‐carcass diversity of Salmonella among various PLN; and 2) characterize the duration of infection of Salmonella within PLN of cattle following intradermal inoculation.
Methodology
To accomplish objective 1, a fecal swab and six PLN were collected from 100 carcasses. Lymph nodes included the left and right subiliac, superficial cervical, and popliteal nodes. Samples were processed to recover Salmonella isolates. In addition, methods were used to quantify the concentrations of Salmonella within PLN.
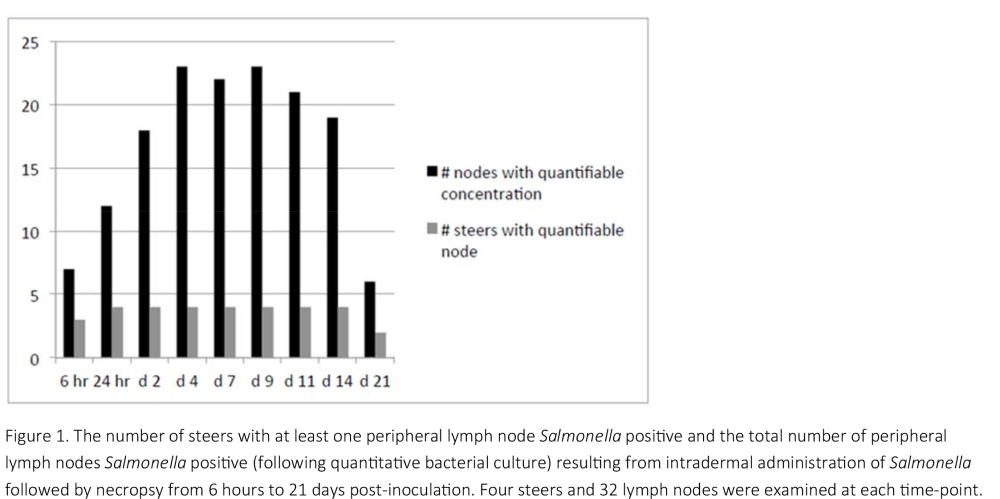
To accomplish objective 2, a recently developed transdermal experimental challenge model was used to determine the duration of infection of Salmonella in the PLN of steers. Thirty‐six Holstein steers (avg. BW = 137 kg) were inoculated with Salmonella (d0), transdermally on each lower leg, and both sides of the back and paunch. Calves (n = 4/time point) were euthanized beginning at 6 h and subsequently on each of days 1, 2, 4, 7, 9, 11, 14 and 21 post‐inoculation. The following PLN (right and left) were collected and cultured (quantitatively and qualitatively) for the challenge strain of Salmonella: subiliac, popliteal, superficial cervical, and axillary.
Findings
In objective 1, Salmonella was recovered from 32 and 80% of the PLN and fecal swabs respectively. The likelihood of recovery of Salmonella decreased over time in that across the sample collection days of 03OCT, 17OCT, 24OCT, and 31OCT, Salmonella was recovered from 58.9, 56.1, 15.8, and 9.1% of PLN, respectively. Further, Salmonella was recovered from 80, 100, 95 and 47.8% of fecal swabs, respectively. Salmonella was recovered from at least one PLN per carcass 100, 96.7, 50, and 37.1% of the time, respectively. Saliently, during the first 2 collection days, Salmonella was recovered from all 6 PLN in excess of 20% of the carcasses.
In objective 2, lymph nodes were generally 100% positive following qualitative culture at 24 hours post‐inoculation and remained so until day 14. By day 21, the percentage of Salmonella positive nodes decreased to approximately 50%, indicating the PLN were starting to eliminate the challenge strain. The exception were the axillary lymph nodes, the majority of which were Salmonella positive at the first necropsy, but sporadically positive throughout the remainder of the experimental period. The implication of this is unclear and warrants further evaluation.
Based on previous research with this experimental model, it was expected that the DOI would last approximately 10 to 14 days. Due to the successful challenge that provided quantifiable concentrations, the DOI was much longer. This indicates that DOI is partly a function of the underlying concentration within the nodes. Under these experimental conditions, elimination from the nodes appears to begin somewhere between days 14 and 21 and it is yet unknown at what time post‐inoculation it will be complete.
Implications
The data reported here in provide important new information on the pre‐harvest ecology of Salmonella within groups of cattle and in particular, within their PLN. One of the key findings is that Salmonella can be widely dispersed across many PLN within a carcass. This highlights the limitations of in-plant controls in that it is not practical or may even be unachievable to remove all PLN given the number and distribution. The need for effective pre‐harvest controls is clear.
To this end, it was possible to provide important new information on the duration of infection. Based on prior research, this appears to be somewhat concentration dependent but, saliently, it is not overly long in that there should be expected quantifiable concentrations to be eliminated soon after day 21. The implication of this is that the observed prevalence of Salmonella within PLN in cattle presented for harvest can be reduced if an intervention – a vaccine or probiotic for example – effectively reduces the DOI. Another opportunity for control are interventions that reduce the rate of new infections.