Project Summary
Evaluating the Impact of Pre- and Post-harvest Process Controls and Intervention Strategies Used to Reduce Escherichia coli O157:H7
- Principle Investigator(s):
- Ashley N. Haneklaus, Gary R. Acuff, and Kerri B. Harris
- Institution(s):
- Texas A&M University
- Completion Date:
- May 2011
Background
Since the 1992 outbreak associated with Escherichia coli O157:H7 contaminated ground beef, the beef industry, researchers, and regulatory agencies have invested a significant amount of money and time trying to prevent E. coli O157:H7 to protect consumers. While the industry has developed and implemented antimicrobial interventions to be applied at various steps during harvest and fabrication, it still struggles with E. coli O157:H7 recalls and outbreaks. Due to the number of recalls and illnesses associated with E. coli O157:H7 over the past couple of years, as well as the increased concern with non-O157 Shiga-toxin producing E. coli serogroups (STECs), it is imperative that a more intensive and coordinated effort be taken to reduce the public health risks associated with beef products. Researchers often focus on a single sector, such as harvest, rather than looking at the total system from pre-harvest, through harvest and to further processing establishments. Multiple variables (e.g., incoming microbial load, dressing procedures, types of interventions being applied, processing and sanitation practices) may impact the contamination levels on the finished products. Therefore, this multi-phase project was designed to evaluate existing pre- and post-harvest process controls and intervention strategies used to reduce E. coli O157:H7, as well as to evaluate the impact of changing/modifying pre- and post-harvest process controls and intervention strategies used to reduce E. coli O157:H7.
The objectives of this study were to:
- To evaluate existing pre- and post-harvest process controls and intervention strategies used to reduce E. coli O157:H7.
- To evaluate the impact of changing/modifying pre- and post-harvest process controls and intervention strategies used to reduce E. coli O157:H7.
Methodology
The present study was conducted in a series of 5 trials. Each trial was executed after a detailed meeting between the beef harvest facilities and the study investigators. Each meeting was utilized to discuss issues/questions that should be addressed in relation to the study objectives, sampling techniques, sampling locations (in-plant and carcass surface locations), and other decisions that were made following data analysis from each trial.
Sampling methods
Exact sample size areas varied by trial. All sponges (3M, St. Paul, MN) used for the study were hydrated with 25 mL of buffered peptone water (BPW; BD Diagnostics, Spark, MD). Gloves were worn at all times, and although they did not need to be sterile, if gloves contacted any surface (carcasses, equipment, sample sponge, etc.) they were changed. Sponges were wrung-out in the bag to remove excess BPW, removed from the bag, and used to swab the sample surface (hide, carcass, processing area, etc.).
When possible, samples were taken along or near the pattern opening lines of the beef carcasses. Irrespective of sample size or location, all samples were taken using 5 horizontal passes, flipping the sponge over, and conducting 5 vertical passes. Samples were stored in insulated coolers with ice packs and transported to the Texas A&M University Food Microbiology Laboratory (College Station) for analysis within 6 h of sample acquisition.
Microbiological analyses
Upon arrival to the laboratory, samples were pummeled by hand for 1 min. Pummeled samples then were plated onto Petrifilm plates by using appropriate serial dilutions and pipetting 1 mL of sample onto the center of the bottom film. For all trials, samples were plated on Aerobic Plate Count (APC) and E. coli/coliform Petrifilm plates. Only select trial samples were plated on Enterobacteriaceae Petrifilm plates. When necessary, a spreader was used over the top film of the Petrifilm plates to distribute the inoculum over the circular area before gel was formed. Plates were incubated for 48 h at 25°C for APC and for 24 h at 35°C for E. coli/coliform and Enterobacteriaceae. Following incubation, plates were counted.
Findings
Data from the present study support the concept that pre- and post-harvest practices may impact pathogen contamination on beef carcasses. Data from both establishments used in this study demonstrated incoming bacterial loads; however, the resulting carcass surface levels were below detection in only one of the establishments. Differences in sanitary dressing practices and other in-plant interventions may contribute to these differences.
Implications
While the beef industry has developed and implemented antimicrobial interventions to be applied at various steps during cattle feeding, harvest, and fabrication, it still struggles with E. coli O157:H7 recalls and outbreaks. Although the efficacy of most antimicrobial interventions has been demonstrated in a laboratory setting, the results obtained in-plant vary, and are sometimes less effective than expected. There are multiple variables (e.g., feedlot interventions, incoming microbial load, dressing procedures, types of interventions being applied in-plant, processing and sanitation practices) that may impact food safety. This study will provide the beef industry with the insight necessary to evaluate and understand how the wide variety of animal, processing establishment, and microorganism variables impact the overall success of a food safety system in reducing or controlling pathogen contamination on beef carcasses.
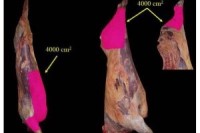


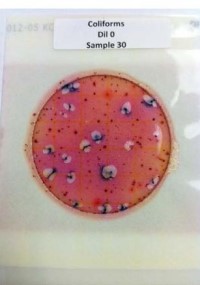
From Left to Right:
Figure 1. Sampling area.
Figure 2. Preparing serial dilutions for plating samples on APC and E. coli/Coliform Petrifilm plates.
Figure 3. Using a spreader to distribute the inoculum in the circular area of the APC and E. coli and Coliform Petrifilm plates.
Figure 4. E. coli/Coliform Petrifilm plate post-inoculation.