Project Summary
The Effect of Lactic Acid and Cooking on the Survivability of E. coli O157:H7 in Needle Tenderized Beef Steaks Managed Under Simulated Industry Conditions
- Principle Investigator(s):
- Chance Brooks, Mindy Brashears, Mark Miller, Alejandro Echeverry, and Cassandra Chancey
- Institution(s):
- Texas Tech University
- Completion Date:
- 2010
Background
E. coli O157:H7 used to only be of concern with ground beef. Recently, the concern has changed to any meat that has been tenderized or injected, as was found in the case with tenderized frozen steaks that were sold to consumers. On August 20, 2004, approximately 406,000 pounds of frozen beef products were recalled due to E. coli O157:H7 contamination. On May 11, 2007, another recall occurred. The items included: strip loins steaks, ball tip steaks, top butt steaks, and sirloin steaks. All of these steaks were mechanically tenderized and linked to illnesses resulting from E. coli O157:H7. These two outbreaks document that E. coli O157:H7 can be transferred into the steaks when they are mechanically tenderized. Because of this, the U.S. Food Safety Inspection Service (FSIS) currently recommends that mechanically tenderized steaks be cooked to a minimum of 68.3°C for 15 seconds to ensure that E. coli O157:H7 bacteria cells have been killed. Therefore, it is important to document that cooking to 68.3°C for 15 seconds is sufficient to kill E. coli O157:H7 cells in mechanically tenderized products.
Lactic acid is used as an antimicrobial to control or reduce bacterial contamination. One bacterium that lactic acid helps to control is E. coli O157:H7. Lactic acid was first discovered by Swedish chemist Carl Wilhelm Scheele in 1789 while investigating the souring of burgundy wine. Since then, scientists have found that lactic acid helps in controlling different bacteria. Most facilities use lactic acid as a critical control point (CCP) in their HACCP plans.
The objective of this research was to characterize the effects of lactic acid spray on needle-tenderized beef strip loins inoculated with low and high levels of Escherichia coli O157:H7 prior to cooking to various endpoint temperatures.
Methodology
USDA Standard strip loins (n = 40) were obtained from a commercial processing facility and transported to the BSL-II pathogen processing facility at Texas Tech University in Lubbock, TX. The strip loins were randomly allotted to two trials. Trial 1 included 20 strip loins inoculated with a 105 CFU/cm2 E. coli O157:H7 cocktail (High inoculation), with an actual attachment rate of 103 CFU/cm2. Trial 2 followed the same procedure - 20 strip loins inoculated with a 103 CFU/cm2 E. coli O157:H7 cocktail (Low inoculation), with an actual attachment rate of 101 CFU/cm2. Trial 1 and Trial 2 included the inoculation of five strip loins and were replicated 4 times. The E coli O157:H7 cocktail was made with four strands isolated from cattle (ATTC A4 966, A5 528, 966 and A1 920) of E. coli O157:H7 each associated with CDC-documented outbreaks. The strip loins were immersed for approximately 15 seconds per side. The strip loins were then allowed to stand for 10 to 15 minutes at approximately 3°C to allow for E. coli O157:H7 attachment. After attachment, strips were allotted numbers, and approximately one-half of the inoculated strip loins (3 from the low and 2 from the high inoculations, alternating with each rep) were treated with 5% lactic acid using a spray cabinet with a conveyorized wire belt. After treatment with lactic acid, all strip loins (treated and non-treated) were vacuumed packaged and stored at 0 - 4°C for 21 days. On day 21 of storage, control and treated samples were removed from their vacuum bags for mechanical tenderization with a blade tenderization unit. Strip loins were placed external fat side down with needle penetration initiated on the lean side. After tenderization, the strip loins were portioned into 2.54 cm thick steaks, while each steak was identified and individually vacuum packaged. Packaged steaks were then stored at approximately 3°C for 7 d in the absence of light. The needle tenderization equipment was cleaned and sanitized between each strip loin. After the 7 d storage period (28 d after inoculation), steaks were transported to the laboratory under refrigeration for cooking and microbial analysis. The steaks were randomly allotted to five targeted internal temperatures (55, 60, 65, 70, and 75°C) while an additional steak from each strip was not cooked (raw) to determine internal microbial counts. Prior to cooking, steaks were threaded with Type J thermocouple wiring to allow for continuous temperature monitoring. Steaks were grilled on a George Foreman grill to their targeted end point temperatures.
Prior to mechanical tenderization on d 21, both fat and lean surfaces of the strip loins were sampled using sponges moistened with 9 mL of BPW (buffered peptone water). At this point, the samples were spread plated on MacConkey’s agar with a TSA (Triptic Soy Agar) overlay to help the injured E. coli O157:H7 cells to recover and grow. The MacConkey’s agar was mixed with potassium tellurite and novobiocin sodium salt to control background flora growth. Plates were incubated at 37°C for 18 to 24 hours. The colonies of E. coli O157:H7 were then counted.
The microbial profile of the internal portion of each strip loin steaks was measured after cooking to six internal temperature endpoints. The High and Low inoculated subprimal samples were also swabbed 15 min after inoculation with the E. coli O157:H7 cocktail (both fat and lean surfaces) as well as after treatment with 5% lactic acid (again, both fat and lean surfaces were sampled). The samples were plated using MacConkey’s agar, mixed with potassium tellurite and novobiocin sodium salt, and overlaid with TSA. Plates were incubated at 37°C for 18 to 24 hours. The colonies of E. coli O157:H7 were hand counted.
Prior to cooking on d 28 post inoculation, the individual steak surfaces were swabbed before cooking and five minutes after reaching the desired internal cooked temperature. After cooking, a core sample was obtained from each steak. The core sample was ground using a food processor and approximately 142 g of the ground samples was placed into a stomacher bag with 90 mL of added enrichment broth. The samples were then stomached for 2 min and then placed in an incubator at 37°C for 18 to 24 hours. After enrichment, samples were analyzed with BAX system to test the presence of E. coli O157:H7.
Findings
Subprimals High Inoculation (105 log CFU/cm2) of E. coli O157:H7:
Results indicate lactic acid treatment decreased (P < 0.05) E. coli O157:H7 bacteria levels on the surface of beef subprimal on d 0 of storage by approximately 1 log CFU/cm2. Data indicate E. coli O157:H7 levels differed on d 0 depending on surface type (lean vs. fat surface) for control and treated subprimals, but the values did not differ statistically. E. coli O157:H7 levels on the lean and fat surfaces of beef subprimals were decreased after 21 d of vacuum package storage at refrigerated temperatures. After 21 d of storage, E. coli O157:H7 levels on the surface of beef subprimals were similar for control-lean surface, control-fat surface and treated lean surfaces. However, E. coli O157:H7 levels were lower (P < 0.05) on surfaces covered in fat than all other treatments after 21 d of storage. These data indicate 5% lactic acid and storage for 21 d in vacuum packaging were effective in reducing E. coli O157:H7 bacteria on the surface of beef subprimals. The combined use of 5% lactic acid and 21 d vacuum packaged storage of subprimals deceased E. coli O157:H7 by approximately 2 log CFU/cm2, regardless of surface type. The effect of storage on E. coli O157:H7 levels could be due to the lack of oxygen during storage, background flora, and/or the cold storage temperatures (0 to 4°C).
Subprimals Low Inoculation (103 log CFU/cm2) of E. coli O157:H7:
Data indicate 5% lactic acid treatment reduced (P < 0.05) E. coli O157:H7 levels on the lean and fat surfaces of beef subprimals stored for 0 d, compared to control lean and fat surface samples. Analysis also indicated storage of beef subprimals in vacuum packaging for 21 d reduced (P < 0.05) E. coli O157:H7 on the lean and fat surfaces of beef subprimals compared to E. coli O157:H7 levels observed on d 0 of storage. The lean side of beef strip loins had lower E. coli O157:H7 levels on day 0 lactic acid treated samples compared to controls (0.43 vs. 1.23 logs, respectively). From day 0 to day 21 there was a reduction on lean surface E. coli O157:H7 counts for the control (from 1.23 logs to 0.16 logs) as well as lactic acid treated strip loins (from 0.43 logs to 0.10 logs). On day 21, there was no significant difference in the amount of E. coli O157:H7 found on the control and lactic acid treated strip loins. E. coli O157:H7 levels on the on the fat side of beef subprimals was greatly reduced (1.26 logs) on day 0 with the lactic acid spray when compared to the control strip loins. At day 21, it was found that the level of E. coli O157:H7 on the lactic acid treated strip loins increased 0.23 logs from day 0, while E. coli O157:H7 levels on the controls decreased (from 1.29 logs to 0.63 logs). However, the lactic acid treated samples at 21 d of storage were still lower than the control strip loins (0.63 logs vs. 0.27 logs for the control and lactic acid treated samples, respectively). Finally, these data indicate higher counts of E. coli O157:H7 were found on the fat side of beef subprimals when compared to the lean side of beef subprimals.
Steaks High Inoculation (105 log CFU/cm2) of E. coli O157:H7:
E. coli O157:H7 levels on the surface of beef steaks portioned from high inoculation subprimals treated with lactic acid prior to needle tenderization and portioning on d 21 of storage are presented in Figure 3. The data indicate steaks from subprimals treated with lactic acid had decreased (P < 0.05) E. coli O157:H7 surface levels compared to the control steaks. Least squares means indicated control steaks had 1.58 log CFU/cm2 E. coli O157:H7 on the surface, while the steaks from lactic acid treated subprimals had 1.2 log CFU/cm2.
The proportion of surface swab samples positive for E. coli O157:H7 after the cooking of steaks taken from high inoculated beef subprimals are presented in Figure 4. The data indicate cooking destroyed E. coli O157:H7 surface counts on the steaks for all samples except one. The positive sample was found among steaks cooked to an internal temperature of 55°C after samples were enriched and analyzed with the BAX system. Although great efforts were taken, this positive sample was likely the result of cross contamination rather than treatment.
The proportion of internal steak samples from high inoculated subprimals positive for E. coli O157:H7 after cooking to various internal temperatures and sample enrichment are presented in Figure 5. There were a total of 11 samples that were found to be positive for E. coli O157:H7, with eight (8) of those samples among the raw steak samples. A single sample (0.05 proportion or 5% of samples) was found to be positive for E. coli O157:H7 after enrichment for each of the following internal steak temperature cooking treatments: 55°, 60°, and 70°C.
Steaks Low Inoculation (103 log CFU/cm2) of E. coli O157:H7:
On day 28 of storage, surface swabs were taken on all steaks prior to cooking. The samples from the low inoculated beef subprimals were enriched prior to analysis with the BAX system to determine E. coli O157:H7 positive samples because no colonies were found on plates.
The proportion of surface swabs on steaks from low inoculated subprimals are presented in Figure 6 and stratified according to designated internal cook temperature treatment group. The data indicate that 35 to 65% of surface samples were positive (after enrichment) for E. coli O157:H7 after 28 days of storage in vacuum packaging. Surface swabs taken from these steaks after cooking produced no E. coli O157:H7 positive results after enrichment and BAX system analysis (data not presented in tabular or graphic form).
The proportion of internal steak samples from low inoculated subprimals positive for E. coli O157:H7 after cooking to various internal temperatures and sample enrichment are presented in Figure 7. Data analysis indicated that only raw steak samples (not cooked; NC) were positive (25% of samples) for E. coli O157:H7. Moreover, data analysis indicated the internal portion of steaks cooked to an internal temperature of 55, 60, 65, 70 or 75°C from low inoculated subprimals were devoid of E. coli O157:H7 after samples were plated, as well as enriched and analyzed with the BAX system.
Implications
USDA-FSIS mandates non-intact beef steaks must be cooked to a minimum of 68.3°C for 15 seconds to ensure that E. coli O157:H7 bacteria cells have been killed. This project indicates that at low inoculation levels (those typical of industry contamination levels) 5% lactic acid treatment, vacuum packaging, refrigerated storage and cooking reduced E. coli O157:H7 to non-detectable levels in needle tenderized beef steaks cooked to 55°C or higher internal temperatures.
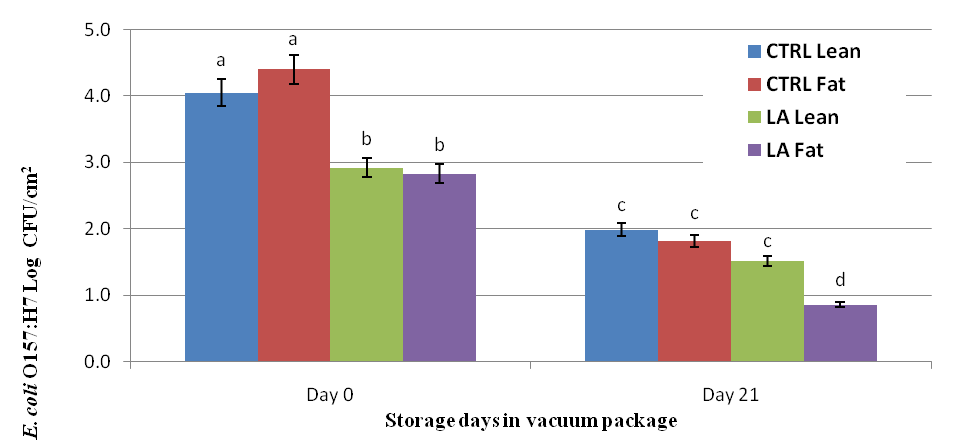
Figure 1. Effect of Lactic Acid (LA) compared to no intervention (CTRL) applied to the lean and fat surface of beef strip loins inoculated with 105 log CFU/cm2 E. Coli O157:H7 and stored in vacuum packages for 0d and 21d. abcd Least square means within storage time of different subscripts differ (P < 0.0009).
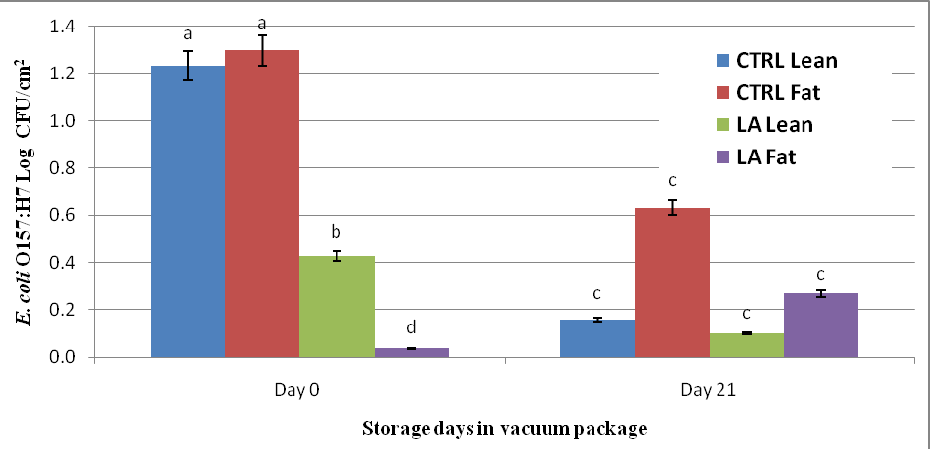
Figure 2. Effect of Lactic Acid (LA) compared to no intervention (CTRL) applied to the lean and fat surface of beef strip loins inoculated with 103 log CFU/cm2 E. coli O157:H7 and stored in vacuum packages for 0d and 21d. abcd Least square means within storage time of different subscripts differ (P < 0.0001).
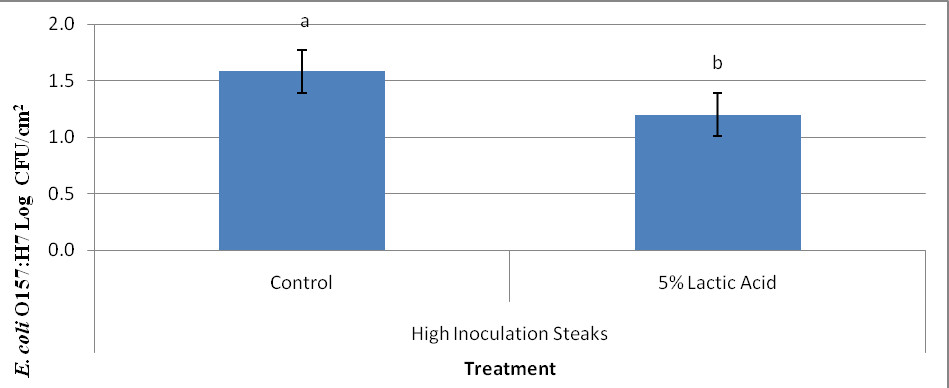
Figure 3. Surface bacterial counts (Log CFU/cm2) of beef strip loins steaks from subprimals inoculated with 105 log CFU/cm2 E. coli O157:H7, that were subjected treatment (lactic acid vs. control) and to needle tenderization prior to portioning (Raw). ab Least square means within storage time of different subscripts differ ( P < 0.0093).
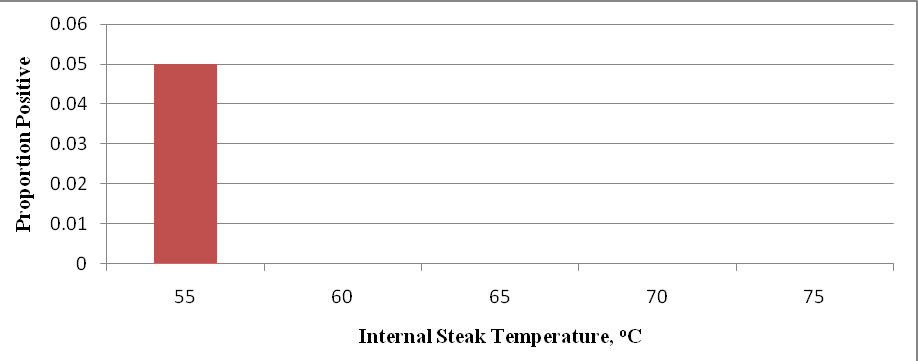
Figure 4. Proportion of steak surface swabs positive for E. coli O157:H7 inoculated steaks cooked to various internal temperatures. Beef strip loins were inoculated on day 0 with 105 log CFU/cm2 of E. coli O157:H7 and treated by means of mechanical needle tenderization and cut into steaks on day 21 and stored additional 7d. For each temperature, individual steaks (n=20) were analyzed and surface swabs taken after cooking.
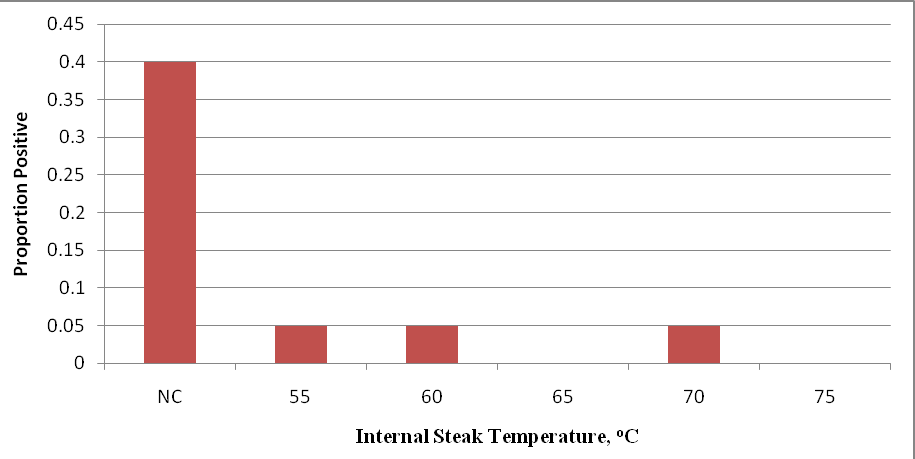
Figure 5. Proportion of internal steak samples positive for E. coli O157:H7 inoculated steaks after 28 days of storage under refrigerated conditions. Beef strip loins were inoculated on day 0 with 105 log CFU/cm2 of E. coli O157:H7 and treated by means of mechanical needle tenderization and cut into steaks on day 21. For each temperature, individual steaks (n=20) were analyzed and meat samples (25 g) taken after cooking (meat samples analyzed with BAX system after cooking to various internal steak temperatures).
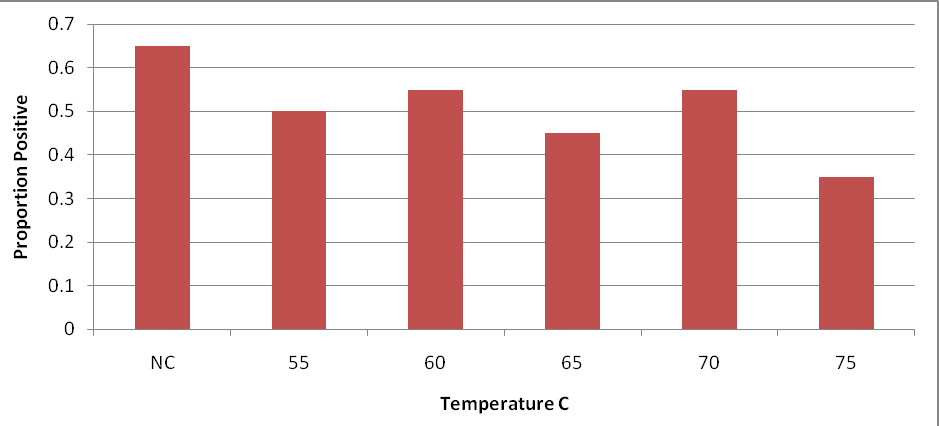
Figure 6. Proportion of steak surface swabs positive for E. coli O157:H7 inoculated steaks cooked to various internal temperatures. Beef strip loins were inoculated on day 0 with 103 log CFU/cm2 of E. coli O157:H7 were subjected to treatment (lactic acid vs. control), needle tenderization, cut into steaks on day 21, and stored an additional 7 days. For each temperature, individual steaks (n=20) were analyzed and surface swabs taken before cooking was done.
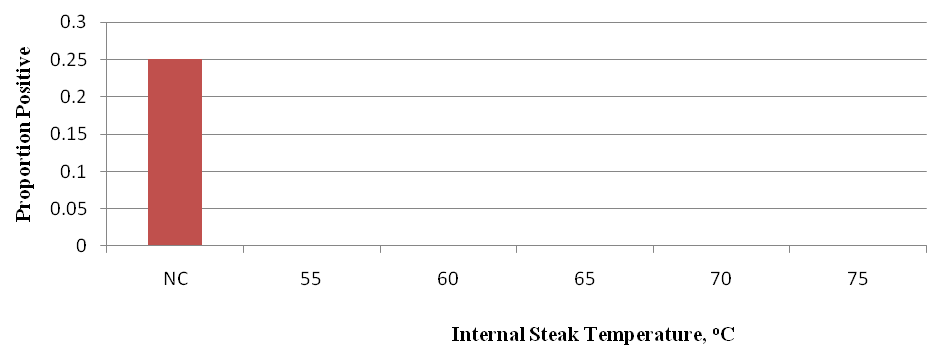
Figure 7. Proportion of internal steak samples positive for E. coli O157:H7 inoculated steaks after 28 days of storage under refrigerated conditions. Beef strip loins were inoculated on day 0 with 103 log CFU/cm2 of E. coli O157:H7 and treated by means of mechanical needle tenderization and cut into steaks on day 21. For each temperature, individual steaks (n=20) were analyzed and meat samples taken after cooking (meat samples analyzed with BAX system after cooking to various internal steak temperatures).